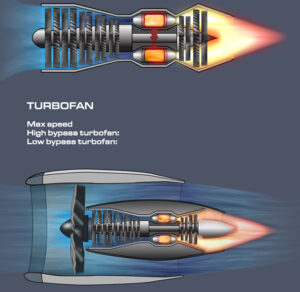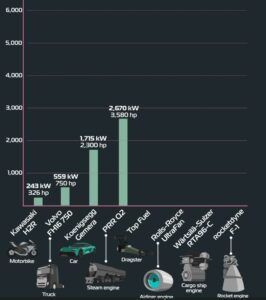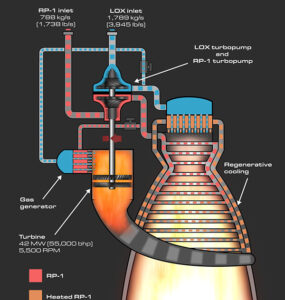
Modern society is built around electricity. It powers almost everything we use on a daily basis.
But what is it exactly?
Electricity is a phenomenon that can be a little tricky to understand. This animated infographic is the first of a series that explains what electricity is and the basics of electrical engineering.
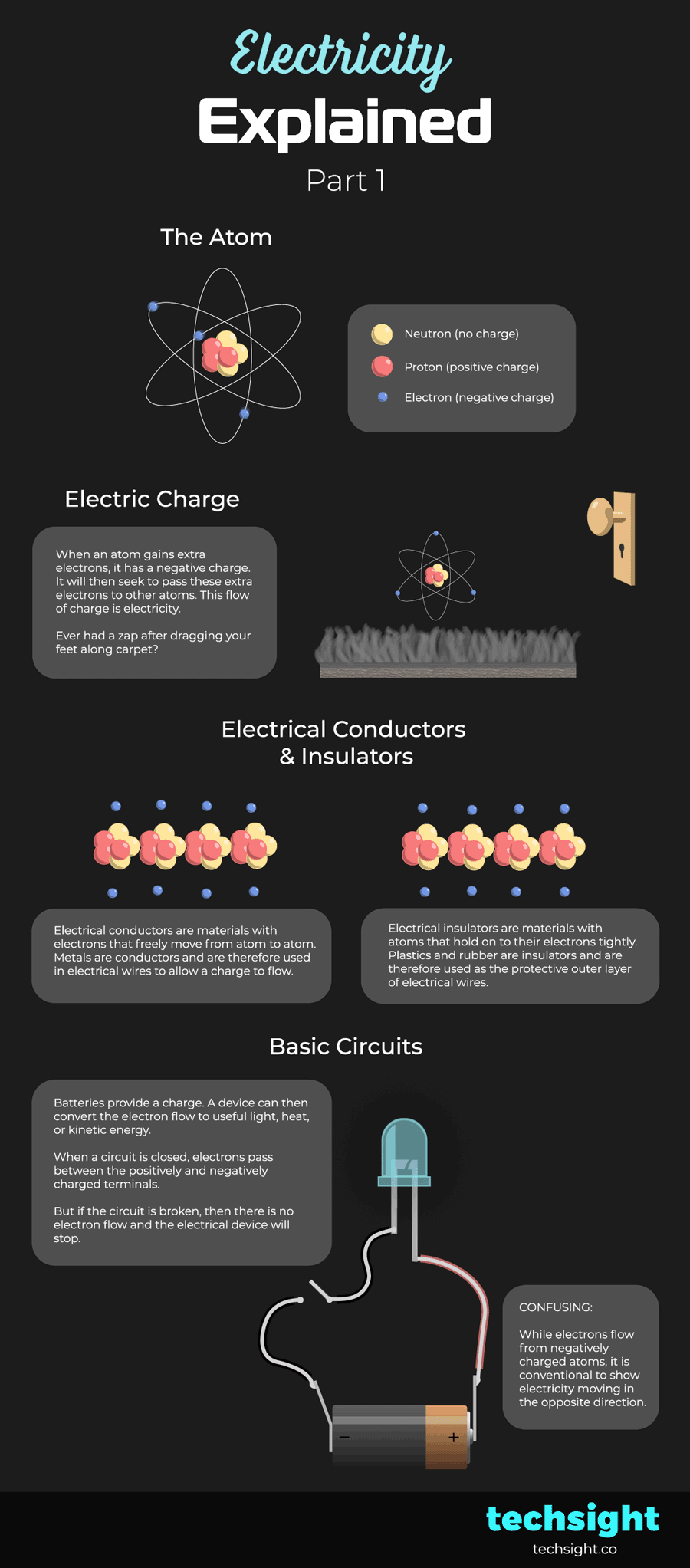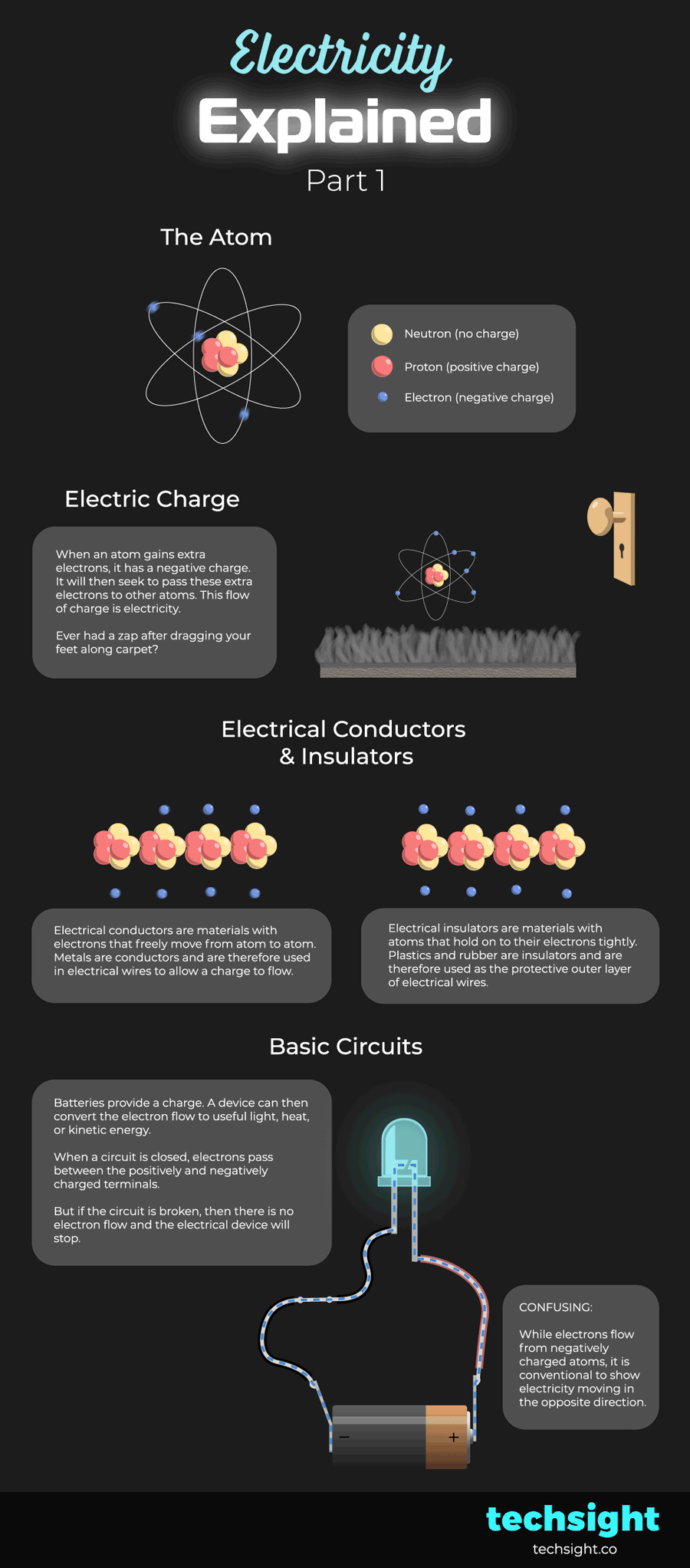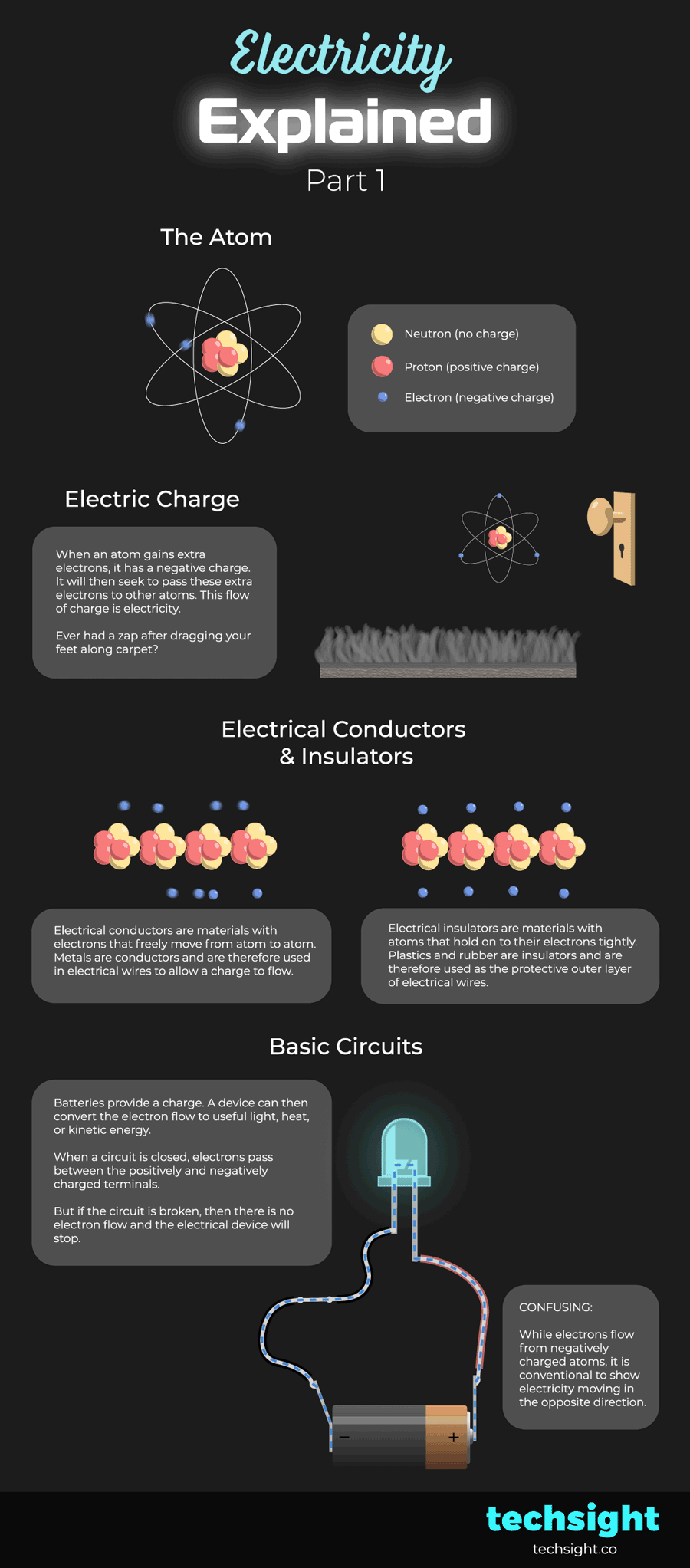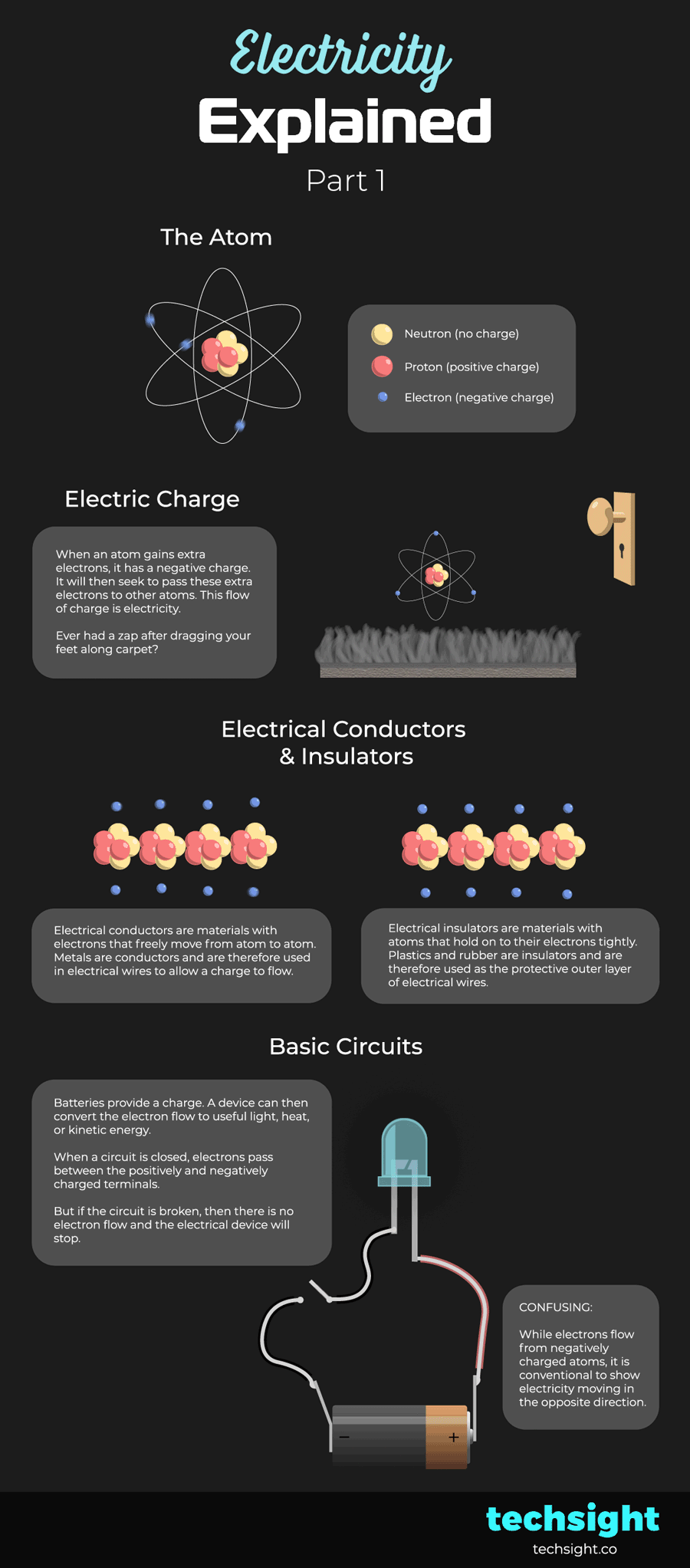
The atom
Atoms consist of:
- Neutrons which have a neutral charge.
- Protons which have a positive charge.
- Electrons which have a negative charge.
Electric Charge
When an atom gains extra electrons, it has a negative charge. It will then seek to pass these extra electrons to other atoms. Atoms that have lost electrons have a positive charge and more readily accept new electrons.
This flow of charge is electricity.
Ever had a zap after dragging your feet along carpet? This is caused by atoms in your body picking up extra electrons from the carpet, and then releasing them when you come in contact with another object. The discharge of electrons is the zap.
Electrical Conductors & Insulators
Electrical conductors are materials with electrons that freely move from atom to atom. This makes it easy for an electrical charge to flow. Metals are conductors and are therefore used in the core of electrical wires.
Electrical insulators are materials with atoms that hold on to their electrons tightly. This makes it hard for an electrical charge to flow. Plastics and rubber are insulators and are therefore used as the protective outer layer of electrical wires.
Basic Circuits
Batteries provide a charge. A device can then convert the electron flow to useful light, heat, or kinetic energy.
When a circuit is closed, electrons pass between the positively and negatively charged terminals.
But if the circuit is broken, then there is no electron flow and the electrical device will stop.
CONFUSING: While electrons flow from negatively charged atoms, it is conventional to show electricity moving in the opposite direction. In other words, electricity flows from the positive terminal of a battery to the negative terminal.